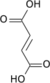
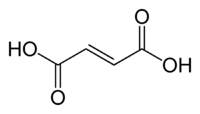
Fumaric acid
| Fumaric acid | |
|---|---|
 |
|
 |
|
|
(E)-Butenedioic acid
|
|
|
Other names
trans-1,2-Ethylenedicarboxylic acid
2-Butenedioic acid trans-butenedioic acid Allomaleic acid Boletic acid Donitic acid Lichenic acid |
|
| Identifiers | |
| CAS number | 110-17-8 |
| EC number | 203-743-0 |
|
SMILES
OC(=O)C=CC(=O)O
|
|
| Properties | |
| Molecular formula | C4H4O4 |
| Molar mass | 116.07 g/mol |
| Appearance | White solid |
| Density | 1.635 g/cm³, solid |
| Melting point |
287 °C |
| Solubility in water | 0.63 g/100 mL |
| Acidity (pKa) | pka1 = 3.03, pka2 = 4.44 |
| Hazards | |
| EU classification | Irritant (Xi) |
| R-phrases | R36 |
| S-phrases | (S2) S26 |
| NFPA 704 |
 1
2
0
|
| Related compounds | |
| Related carboxylic acids | maleic acid succinic acid crotonic acid |
| Related compounds | fumaryl chloride fumaronitrile dimethyl fumarate iron(II) fumarate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid, wherein the carboxylic acid groups are cis. It has a fruit-like taste. The salts and esters of fumaric acid are known as fumarates.
Fumaric acid, when added to food products, is an acidity regulator denoted by E number E297.
Contents |
Biology
Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Iceland moss.
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food. It is formed by the oxidation of succinate by the enzyme succinate dehydrogenase. Fumarate is then converted by the enzyme fumarase to malate. Human skin naturally produces fumaric acid when exposed to sunlight.
Fumarate is also a product of the urea cycle.
Medicine
Fumaric acid esters are sometimes used to treat psoriasis, as it has been suggested that the condition is caused by an impairment of fumaric acid production in the skin.[1] A starting dose is 60-105 mg daily, which may be gradually increased to as much as 1,290 mg per day. Side-effects include kidney or gastrointestinal disorders, as well as skin flushing; these are mainly caused by excess intake. Decreased white blood cell (WBC) counts have been reported with prolonged use.
Food
Fumaric acid is a food acidulent used since 1946. It is non-toxic. It is generally used in beverages and baking powders for which requirements are placed on purity. It is generally used as a substitute for tartaric acid and occasionally in place of citric acid, at a rate of 1.36 g of citric acid to every 0.91 grams of fumaric acid to add sourness, similar to the way malic acid is used. It is also used as a coagulant in stovetop pudding mixes.
Chemistry
Fumaric acid was first prepared from succinic acid.[2] A traditional synthesis involves oxidation of furfural (from the processing of maize) using chlorate in the presence of a vanadium-based catalyst.[3] Nowadays industrial synthesis of fumaric acid is mostly based on catalytic isomerisation of maleic acid in aqueous solutions at low pH. Maleic acid is accessible in large volumes as a hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane.[4]
The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes additions across the double bond, and it is an excellent dienophile.
Fumaric acid does not combust in a bomb calorimeter under conditions where maleic acid deflagrates smoothly. For teaching experiments designed to measure the difference in energy between the cis- and trans- isomers, a measured quantity of carbon can be ground with the subject compound and the enthalpy of combustion computed by difference.
Other uses
Fumaric acid is used in the manufacture of polyester resins and polyhydric alcohols and as a mordant for dyes.
Safety
Fumaric acid converts to the irritant maleic anhydride, upon partial combustion.
See also
- Dermatology
- Photosynthesis
- Maleic acid, the cis-isomer of fumaric acid
References
- ↑ Br J Dermatol 05; 152(4):597-615), BR J Dermatol 98;138(3): 456-460
- ↑ Volhard, J. "Darstellung von Maleïnsäureanhydrid" Justus Liebig's Annalen der Chemie 1892, volume 268, page 255-6. DOI: 10.1002/jlac.18922680108
- ↑ Milas, N. A. "Fumaric Acid" Organic Synthesis 1943, Collective Volume 2, page 302. Online version
- ↑ British Patent No. 775,912, publicated on the May 29, 1957, by Monsanto Chemical Company.
External links
| Oxaloacetate | Malate | Fumarate | Succinate | Succinyl-CoA | ||||||||||||
| Acetyl-CoA | NADH + H+ | NAD+ | H2O | FADH2 | FAD | CoA + ATP(GTP) | Pi + ADP(GDP) | |||||||||
| + | H2O | NADH + H+ + CO2 | ||||||||||||||
| CoA | NAD+ | |||||||||||||||
| H2O | H2O | NAD(P)+ | NAD(P)H + H+ | CO2 | ||||||||||||
| Citrate | cis-Aconitate | Isocitrate | Oxalosuccinate | α-Ketoglutarate | ||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||